 | . | Step 22 of 41 |
 |
Clinical Stem 1
Non-smoking female with a new right upper lobe lung mass and three PET-positive mediastinal lymph nodes
Non-smoking female with a new right upper lobe lung mass and three PET-positive mediastinal lymph nodes
Answer B
EGFR TKIs administered to Asian patients who were EGFR mutation-positive improved progression-free survival when compared with those who received chemotherapy with carboplatin/paclitaxel. The response rate in mutation-positive patients was 73.7% versus 30.7 % in the chemotherapy group. Progression-free survival was 10.8 months versus 5.4 months, favoring EGFR TKIs.
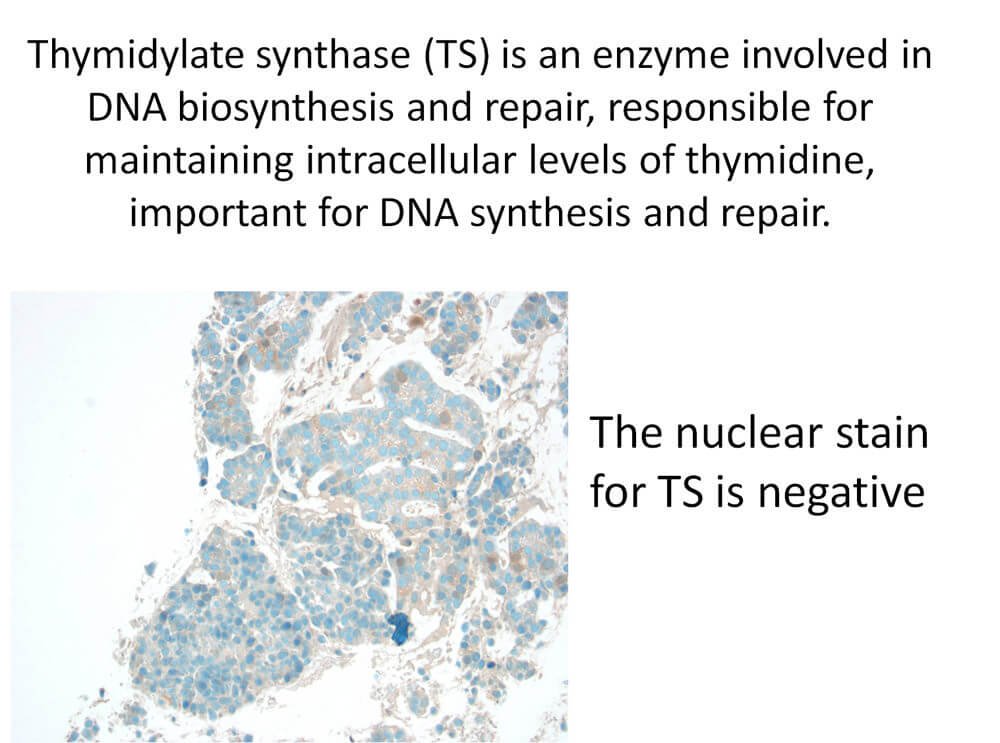
Folate analogue metabolic inhibitors have been studied in NSCLC patients with low expression of thymidylate synthase, also known as TS. This enzyme involved in DNA biosynthesis is responsible for maintaining intracellular levels of thymidine, which is important for DNA synthesis and repair. Immunohistochemistry and RT-PCR on FFPE tissue can be analyzed for TS expression, which is higher in patients with squamous cell carcinoma compared with adenocarcinoma and is correlated with resistance to folate analogue metabolic inhibitors.
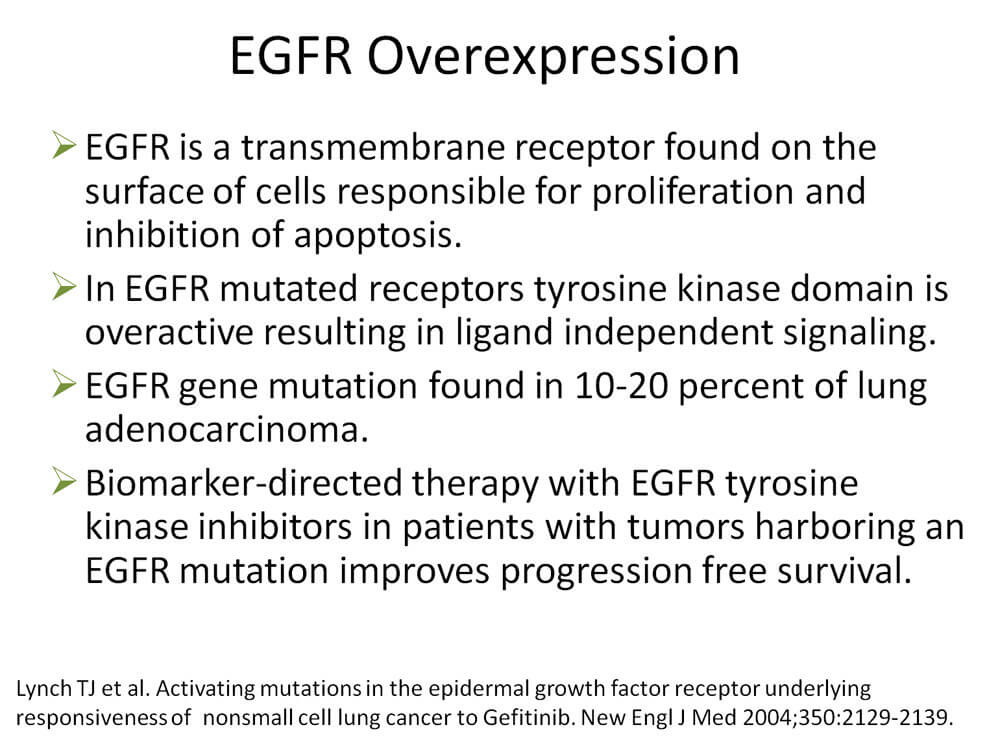
In Caucasians, a 58% objective response rate was reported using EGFR TKIs compared with 15% response following chemotherapy. Progression-free survival improved from 5.2 months to 9.4 months. Because our patient tested positive for EGFR mutation, initiation of EGFR TKI is appropriate.
References:- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010; 363:1693-1703.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361: 947-57.
- Maemondo M, Inoue A, Kobayashi K, et al; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-8.
- Rosell, R. Gervais R, Vergnenegre A, et al. Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. J Clin Oncol 29: 2011 (suppl; abstr 7503).
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26:3543-3551.







